At IBCR we care for sponsor needs and understand sponsor’s requirement. That's why we've structured IBCR to be client-centric in all that we do. Clinical management services are at our nucleus and the supporting functional areas serve into the teams that manage and conduct client projects. Our people are keen in clear accountability for overall outcomes and deliverables. IBCR offers a comprehensive suite of clinical services to meet your needs. IBCR believes to network with atleast 10000 and more clinical investigators across the globe to contribute its business model.

IBCR offers the following beneficial strategies in clinical trial business through its various business models.
IBCR - CIRMO
IBCR- SATAPP - To ensure timely completion of patient inclusion in the study
IBCR- ECONO - To cut down the operational cost up to 50%
IBCR- FASTA-DM - To achieve data base lock (DBL) within 48 hrs of LPLV.
IBCR- XPSV – A different outlook for trial monitoring.
IBCR- PRAWN – Pharmaceutical Research Activity Webbing Network.
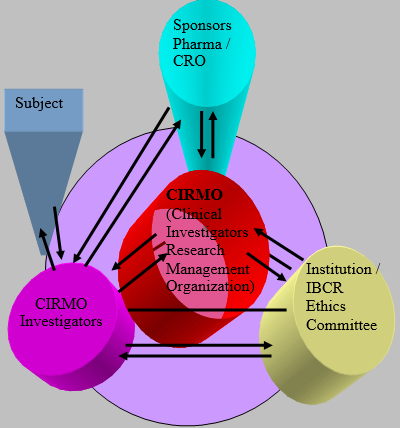
Clinical Investigator’s Research Management Organization (CIRMO)
A Site Management Organization (SMO) performs and manages research for individual Investigative sites. Some pharma companies and CRO had come up with a concept of central SMOs' which coordinate the project execution activities of sponsor / CRO at its operational sites. In the entire clinical trail model Investigators are independent and not bound to any set up. It is to note that the clinical investigators data base is not made public by sponsors. Most of the sponsor / CRO will do project feasibility in the corporate cities and will give repeated projects to the same investigators. This leads to a situation where investigators will have more than one project for same indication, which hinders the subject recruitment; clinical discretion on patient inclusion and encourage investigators to look upon project with more benefit. Moreover sponsor / CRO would initiate study with certain investigators whom does not have the intended patient population. There are some instances where a clinical investigation team would perform well in one study and null in the subsequent study of same indication due to the lack of structural team work. These investigators are unattended further for clinical research projects or they would not know how to approach a sponsor to express their interest. On the other side many investigators practicing at the second class cities of the country are untouched / identified for research programs. There is also no clinical investigators association to showcase their investigators research activities. Considering these restrictions in the present clinical trial execution at sites the present model is proposed to address the identified issues.
In the present model of Clinical Investigators Research Management Organization (CIRMO) proposed by Institute of Biology and Clinical research (IBCR), the investigators will be networked under one roof as IBCR clinical investigators and the CIRMO of IBCR will get clinical research projects to its investigators from various sponsors/ CRO. CIRMO will train its investigators on various regulations; guidelines and will also assist on project execution activities. This model is robust enough to accommodate maximum sites, sponsors, projects and investigators across globe. This clinical investigation team will be made public which helps sponsors to identify clinical investigators of a region. This model also helps the investigators to discuss project related communications, clinical difficulties and other project queries among themselves without breaking the confidentiality clause. Project execution strategies like faster recruitment of patient can be easily passed on among investigators. Through this model potential investigators from second class cities will be identified and exposed internationally. CIRMO will also ensure the project flow to right investigators and thus avoid project competency within an investigator.
Claim 1: To associate Clinical Research Investigators (physicians) across globe as IBCR investigators and to canvass sponsor / CRO to avail clinical research projects to IBCR investigators.
Claim 2: Signing of Master confidentiality agreement & Clinical Trial agreement by a competent authority of IBCR shall be considered as signed by all IBCR clinical Investigators associated with the project as the investigators are abide with IBCR SOP’s & service agreement. However the copies of clinical trail agreement with / without the name of investigators shall be filed in all participating sites based on sponsor discretion.
Claim 3: IBCR investigators can associate within themselves for project specific patient referral, satellite sites operation with a central data modeling at the principal site.
Claim 4: If one site of an IBCR investigator is audited for QA, then the subsequent corrective action and preventive action (CAPA) can be implemented immediately to all participating sites across globe. However CIRMO of IBCR shall ensure that a CAPA implementation record from all other participating sites to be forwarded to sponsor QA.

Advantages: Applicable to multiple sites, multiple CRO & Sponsors, multiple Investigators and to multiple project / Investigators are bound to IBCR and can do research only on IBCR projects / Data modeling (credibility / consistency & Integrity of data can be achieved) / IBCR is independent to sponsor / CRO. IBCR trained investigators can be utilized for years / No site training costs to sponsors/ Clinical trial execution can be done with one SOP across globe / The QA audit & subsequent corrective action and preventive action (CAPA) can be implemented immediately to all participating sites across globe / Flexible to work with multiple sponsor SOP’s.